About clinical trials

- Clinical Trials
- About clinical trials
About clinical trials
Clinical trials are a set of procedures conducted to examine a substance’s clinical safety, efficacy and side effects in human before a new drug comes into the market. In addition, other pharmacological information is investigated such as drug absorption, distribution, metabolism, and excretion. Once a substance has successfully proved its safety and efficacy through multiple clinical trials, a new drug can be approved by national regulatory authorities for manufacturing. A clinical trial is routinely classified into four different phases depending on the development process. The relationship between clinical trials and new drug development can be demonstrated as below.
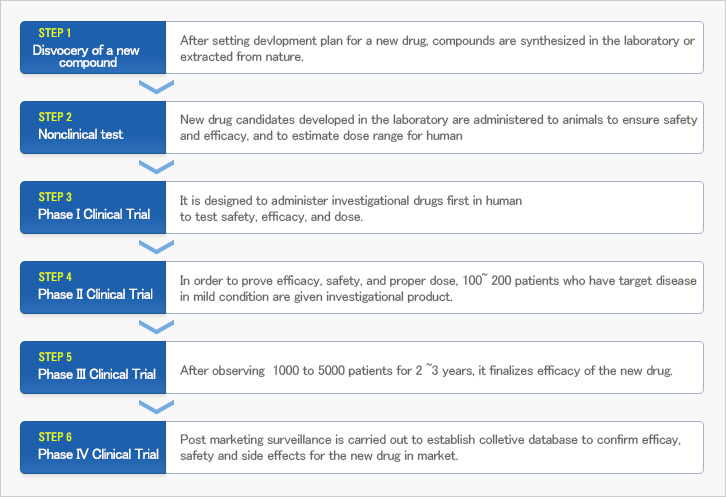
Once a new drug enters into the market, post marketing surveillance is carried out to establish collective and voluminous data base about the new drug. Consequently, all the phases involved in a clinical trial contribute to human welfare and health.
A clinical trial is categorized depending on its type into pharmacological, therapeutic exploration, therapeutic confirmation, and therapeutic treatment. In the early phase of a clinical trial, drug safety and tolerance in a relatively short period are evaluated. Information regarding pharmacokinetics and pharmacodynamics is navigated to determine dosage range and the drug administration schedule
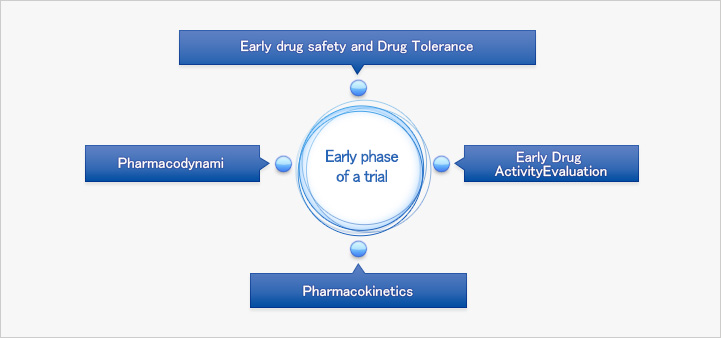
The early phase of clinical trials is designed to administer investigational drugs first in human. In this early phase, it does not aim to test therapeutic clinical evidence, and only healthy volunteers can participate. However, if the drug has serious potential toxicity such as cytotoxic drugs or anticancer drugs, trials will be conducted on only certain patient groups. In order to validate the study’s integrity, randomization and blinding are structurally designed. Following is the list of evaluation that the early phase of the study targets to assess.
Evaluation of Drug safety and tolerance
Drug tolerability and possible adverse events within a predicted range of dosage are observed during an initial stage of a clinical trial. This type of study is usually designed with either single or multiple drug administration during a certain period of time.
Pharmacokinetics
During clinical trials, investigators should be able to describe drug absorption, distribution, metabolism, and excretion. This is one of the major purposes of the early phase of clinical trials. Pharmacokinetic studies can be performed as either independent research or a research in conjunction with drug safety and tolerance. Significance of pharmacokinetics study lies on the evaluation of drug clearance, possibility of accumulated metabolites, or potential drug to drug interaction. It is, also, likely to connect with other clinical trials that are following. Particularly, it is imperative to know how food can influence oral drug’s bioavailability. Study results, especially pharmacokinetics, need to be obtained from patients such as immunosuppressive patients (with impaired kidney or liver function), the elderly, children or infants, women, and certain ethnic groups of people. Drug to drug interaction is important to know as well. This data can be obtained during either the stages following stages of clinical trials, or in vitro study done in a laboratory before clinical trials.
Pharmacodynamic evaluation
Investigators verify pharmacodynamic study, drug concentration study, and pharmacokinetic study, (PK/PD study) in healthy volunteers or in patients. Studies are analyzed in consideration of the type of investigational product and outcome variables. If appropriate measures are available, pharmacodynamic data can assess initial drug activity and underlying efficacy in patients. Additional studies will decide drug usage and optimal dosage.
The initial drug activity evaluation
As a secondary goal, a preliminary study is done for active or potential therapeutic benefits. In general, these studies are accomplished in an additional step. However, the drug activity can be estimated only when short-term drug exposure can regulate it.